Embark on a journey through stoichiometry chapter 11 study guide, where the intricate world of chemical reactions unfolds before your eyes. This comprehensive guide delves into the fundamental concepts, calculations, and applications of stoichiometry, empowering you to navigate the complexities of chemical equations with precision and confidence.
Stoichiometry, the study of quantitative relationships in chemical reactions, provides a systematic approach to understanding the behavior of reactants and products. By unraveling the stoichiometric principles, you will gain invaluable insights into predicting reaction outcomes, optimizing chemical processes, and comprehending the intricate tapestry of chemical transformations.
Stoichiometry Basics
Stoichiometry is the branch of chemistry that involves the study of the quantitative relationships between reactants and products in chemical reactions. It allows us to predict the amount of reactants and products involved in a reaction, as well as the limiting reactant and the theoretical yield.
Stoichiometric calculations are essential for understanding the behavior of chemical reactions and for predicting the outcome of experiments. They are used in various fields, including chemistry, biochemistry, and engineering.
Methods for Balancing Chemical Equations
- Coefficient method: Adjust the coefficients in front of each chemical formula to ensure that the number of atoms of each element is the same on both sides of the equation.
- Half-reaction method: For redox reactions, balance the equation by dividing it into two half-reactions (oxidation and reduction) and balancing each half-reaction separately.
Mole Calculations

The mole is the SI unit of amount of substance. It is defined as the amount of substance that contains exactly 6.02214076 × 10 23elementary entities (atoms, molecules, ions, or electrons).
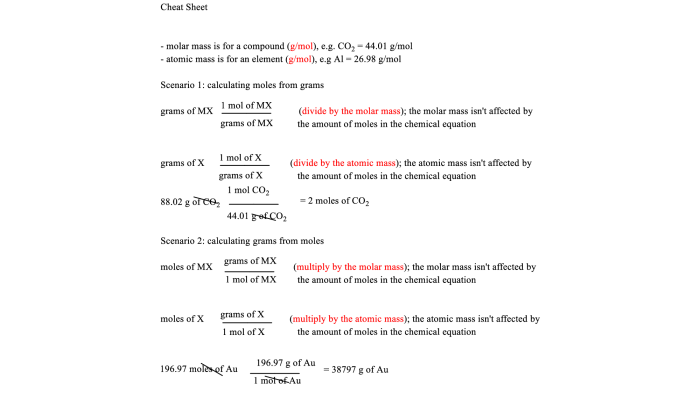
Steps for Converting Between Mass and Moles, Stoichiometry chapter 11 study guide
- Convert the mass to grams (g).
- Find the molar mass of the substance (g/mol).
- Divide the mass by the molar mass to obtain the number of moles.
Limiting Reactants and Excess Reactants: Stoichiometry Chapter 11 Study Guide
In a chemical reaction, the limiting reactant is the reactant that is completely consumed, limiting the amount of product that can be formed. The excess reactant is the reactant that is present in excess, meaning that it is not completely consumed.
How to Identify the Limiting Reactant
To identify the limiting reactant, compare the mole ratios of the reactants to their stoichiometric coefficients. The reactant with the smallest mole ratio is the limiting reactant.
Solution Stoichiometry

Solution stoichiometry deals with the quantitative relationships between reactants and products in solutions.
Molarity and Its Units
Molarity (M) is a measure of the concentration of a solution and is defined as the number of moles of solute per liter of solution.
Gas Stoichiometry
Gas stoichiometry involves the study of the quantitative relationships between reactants and products in gas-phase reactions.
Relationship Between Gas Volume, Pressure, Temperature, and Moles
The ideal gas law relates the volume, pressure, temperature, and number of moles of a gas:
PV = nRT
Redox Reactions
Redox reactions are chemical reactions that involve the transfer of electrons between atoms or ions.
How to Balance Redox Reactions Using the Half-Reaction Method
- Identify the oxidation and reduction half-reactions.
- Balance each half-reaction in terms of mass and charge.
- Multiply the half-reactions by appropriate factors to make the number of electrons lost equal to the number of electrons gained.
- Add the balanced half-reactions to obtain the overall balanced redox reaction.
Key Questions Answered
What is the significance of balancing chemical equations in stoichiometry?
Balancing chemical equations is crucial in stoichiometry as it ensures that the number of atoms of each element on the reactants’ side matches the number of atoms of the same element on the products’ side. This adherence to the law of conservation of mass is essential for accurate stoichiometric calculations and predictions.
How can I determine the limiting reactant in a chemical reaction?
To identify the limiting reactant, compare the mole ratio of each reactant to its stoichiometric coefficient in the balanced chemical equation. The reactant with the smallest mole ratio relative to its stoichiometric coefficient is the limiting reactant, as it will be completely consumed before any other reactant.
What is the role of molarity in solution stoichiometry?
Molarity, expressed in moles per liter (mol/L), is a measure of the concentration of a solution. It represents the number of moles of solute dissolved in one liter of solution. Molarity plays a crucial role in solution stoichiometry, enabling the calculation of the amount of solute or solvent required for a desired reaction or solution preparation.
